Vaccines to tackle the increasing Mpox outbreak in the Democratic Republic of Congo (DRC) and nearby countries may not arrive soon.
The World Health Organisation (WHO) is contemplating categorising the outbreak as an emergency after Africa Centres for Disease Control and Prevention (Africa CDC) declared on Tuesday, August 13,
The day after, a WHO committee convened to evaluate the global risk.
Despite expectations that these steps would stimulate worldwide efforts, challenges such as limited vaccine availability, financial deficits, and ongoing outbreaks remain obstacles.
Jean-Jacques Muyembe-Tamfum, the head of Congo’s Institute National pour la Recherche Biomedicale (INRB), emphasised the urgency of declaring an emergency due to the rapid spread of the disease.
He hopes this will lead to increased funding and improved vaccine access in Congo. Still, he acknowledged the difficulties in a country already burdened by conflict and other diseases.
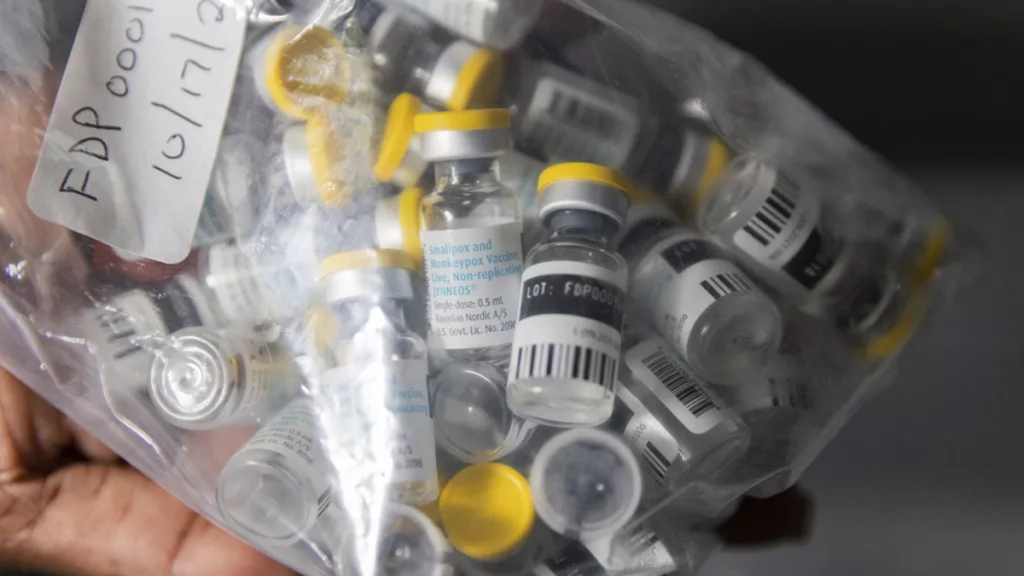
Africa CDC recently secured emergency funding of $10.4 million from the African Union and plans to acquire 3 million vaccine doses this year, although details are scarce. In Congo, only 65,000 doses are expected soon, with vaccination campaigns unlikely to commence before October.
This year, Africa has recorded over 15,000 suspected Mpox cases and 461 deaths, primarily among children in Congo, according to Africa CDC. The typically mild virus can be fatal, causing flu-like symptoms and pus-filled lesions.
A new virus variant has incited outbreaks in eastern Congo refugee camps, spreading to Uganda, Burundi, Rwanda, and Kenya for the first time. Ivory Coast and South Africa are also grappling with outbreaks related to a different strain, which went global in 2022.
Two vaccines, Bavarian Nordic’s Jynneos and LC16 by KM Biologics, were used during that outbreak. However, neither has been available in Congo or Africa, where Mpox has been endemic for decades. Only LC16 is authorised for use in children.
Congo’s regulators endorsed these vaccines in June, but the government has not yet requested them from manufacturers or donors like the United States through Gavi.