The National Agency for Food and Drugs Administration and Control (NAFDAC) has placed a ban on AVEO Pharmaceuticals Pvt Limited over its involvement in the production and illegal exportation of tapentadol to West African countries, including Nigeria.
The pharmaceutical company, based on the outskirts of Mumbai, India, was also linked to the manufacture and unauthorised distribution of high-dose tramadol, particularly the 250mg variant.
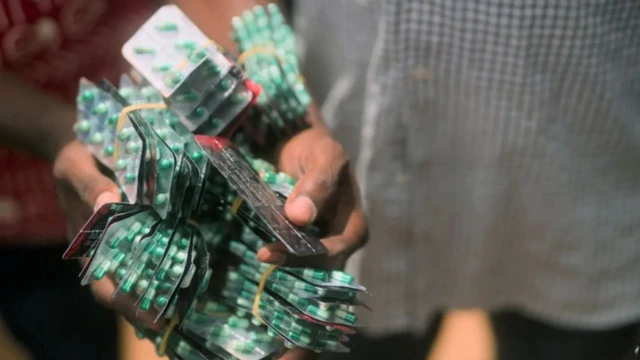
As a result, NAFDAC has prohibited the import and sale of tapentadol and carisoprodol combinations, such as Tafrodol and Royal 225, across Nigeria.
Announcing the development on Friday, NAFDAC Director-General, Prof. Mojisola Adeyeye, stated that AVEO Pharmaceuticals, managed by Vinod Sharma, had been producing and exporting highly addictive pills containing tapentadol, a potent opioid, and carisoprodol, a banned muscle relaxant with severe side effects, including the risk of overdose and death.
She revealed that an undercover investigator, posing as an African businessman looking to supply opioids to Nigeria, secretly recorded Sharma admitting to exporting large quantities of the drugs across West Africa, where they are widely misused as street drugs.
Adeyeye stressed that the combination of these drugs is not licensed for use anywhere in the world and is not registered by NAFDAC, as it poses serious health risks, including breathing difficulties, seizures, and potential fatality.
The agency urged Nigerians to support its fight against substandard and falsified medicines by avoiding unregistered products and only using prescribed medications from certified medical professionals.
“NAFDAC has never registered Tafrodol or Royal 225 or a strength of tramadol greater that 100 mg (the prescription strength), or any product manufactured by Aveo Pharmaceuticals Pvt Limited. Therefore, drawing from the NAFDAC Act Cap N.1 LFN 2004 and the Counterfeit and Fake Drugs and unwholesome Processed Foods (Miscellaneous Provisions) Act Cap C.34 LFN 2004, NAFDAC has decided to BLACKLIST AVEO Pharmaceuticals Pvt Limited. We have also put in place measures to prevent future registration of any product manufactured by this company.
“The public is therefore advised to support NAFDAC’s fight against fake, substandard and falsified pharmaceutical products. They are also advised to avoid the use of unregistered products and consumption of medicines without prescription from trained medical practitioners,” she said.
NAFDAC has now intensified its crackdown on illegal and counterfeit pharmaceuticals, working alongside security agencies to prevent the import and distribution of harmful drugs.
Adeyeye reassured the public that NAFDAC remains committed to ensuring only safe, effective, and high-quality medicines are available in Nigeria.
“This is to assure the public that NAFDAC will continue to deploy various methods to ensure that only quality, safe and efficacious medicines are available for distribution, sale and use within Nigeria,” she stated.


