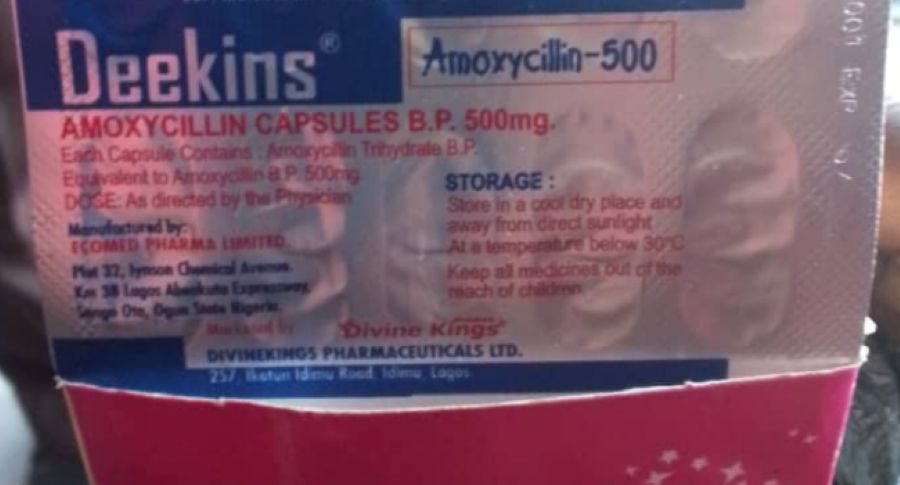
The National Agency for Food and Drug Administration and Control has ordered a recall for a specific batch of Deekins Amoxycillin 500mg Capsules according to a statement posted by the Agency on X on Wednesday.
The batch in question, identified by lot number 4C639001, was produced by Eco-med Pharma Ltd and distributed by DevineKings Pharmaceutical Ltd.
The recall was prompted by reports of serious adverse drug reactions linked to this batch. Eco-med Pharma Ltd said that a hospital had reported three instances of severe reactions in patients who received capsules from this particular batch.
“NAFDAC is alerting the public regarding the recall of one batch of Deekins Amoxycillin 500mg Capsules, created by Eco-med Pharma Ltd and distributed by DevineKings Pharmaceutical Ltd, with lot number 4C639001. “The statement said.
“This batch is being recalled in light of reports of serious adverse drug reactions.
“According to Eco-med Pharma Ltd, a hospital has reported three cases of serious adverse drug reactions in patients who were given capsules from the Deekins Amoxycillin batch.”
NAFDAC urged healthcare professionals and consumers to stop using the affected batch immediately and to report any suspected cases of substandard or fake medications to the closest NAFDAC office.


